views
The market research report for the In-Vitro Diagnostics market includes a prediction for the years up to 2029 as well as in-depth information on the market's size, rate of growth, revenue, trends, and potential. Also carefully analysed are the main variables affecting the development of this sector. This study involved a thorough quantitative examination of the market, and the findings can be used to inform the creation of growth- and productivity-boosting measures.
Request for free Broacher :https://www.maximizemarketresearch.com/request-sample/362
In-Vitro Diagnostics Market Overview:
Focusing on the most recent drivers, restraints, and opportunities for the In-Vitro Diagnostics industry, the in-depth company firm's study is on the In-Vitro Diagnostics market. Discussions with eminent businesses follow to confirm the results. In the secondary review and analysis of the same, both paid and unpaid data sources are utilised. Data on each player's supply and consumption are assembled using official government sources, independent data sources, and, in the case of publicly traded firms, financial reports of the company. Even if financial documents from businesses are not made available to the general public, the tax division of the local government may be able to obtain them.
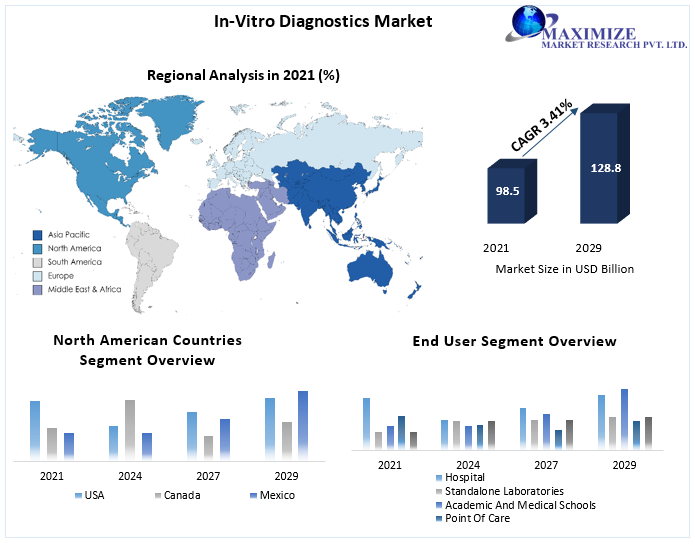
The In-Vitro Diagnostics Market size was valued at USD 98.5 Bn. in 2021 and the total revenue is expected to grow by 3.41 % from 2022 to 2029, reaching nearly 128.8 Bn.
COVID-19 Impact:
The analysis examines the existing and potential impacts of the COVID-19 pandemic on the entire market and delivers more accurate and true estimates in accordance with the market scenario. The world is in anguish due to the coronavirus outbreak. Almost every country has had strict social segregation laws in place, like lockdowns. This has caused disruptions in supply systems. The epidemic has altered societal structures all across the world. The market as a whole is affected by the COVID-19, and as that impact increases in 2019 and 2020, so does the rate of growth. In order to provide decision-makers with reliable data and experiences, businesses and organisations are being compelled by the COVID-19 tragedy to manage risk and digitise operations. This is consistent with the most recent analysis, research, and recommendations from MMR on the management challenges that are crucial for businesses and organisations and have a substantial market impact.
Request for Free sample Pages :https://www.maximizemarketresearch.com/request-sample/362
In-Vitro Diagnostics Market Key players:
• Agilent Technologies (US)
• Bio-Rad Laboratories (US)
• Becton, Dickinson, and Company (US)
• Thermo Fisher Scientific Inc. (US)
• Abbott (US)
• Johnson & Johnson (US)
• QIAGEN (Germany)
• Siemens AG (Germany)
• Roche Diagnostics (Switzerland)
• F. Hoffmann-La Roche Ltd (Switzerland)
• BioMérieux SA (France)
• DiaSorin (Italy)
• Sysmex Corporation (Japan)
Key company and market share insights:
The major competitors are listed here for audiences to learn about. The main strategic actions these businesses take to maintain market dominance are reviewed in this study, including product portfolio strengthening, M&A, partnerships, new, innovative products, and geographic penetration. In addition to critical financial information and current events, the report also provides the company's goals. The companies' global sales, earnings, and profit margins from 2017 through 2022 are also covered in more detail for users.
In-Vitro Diagnostics Market Dynamics:
The MMR report analyzes factors affecting market from both demand and supply side and further evaluates In-Vitro Diagnostics market dynamics effecting the market during the 2021-2029 i.e., drivers, restraints, opportunities, and future trend.
Globally Increasing Rates of Infectious and Chronic Disorders
Increased prevalence of infectious and chronic diseases globally has led to an increase in in-vitro diagnostics. Heart disease, cancer, diabetes, respiratory issues, Alzheimer's, kidney problems, malaria, jaundice, dengue, TB, and other illnesses are among these. For example, the Centres for Disease Control and Prevention (CDC) estimates that 5.9 million Americans will have Alzheimer's disease in 2021. Additionally, over 241 million cases of malaria were found worldwide in 2021. In the US, roughly 2,000 cases of malaria are identified annually. Additionally, the use of cutting-edge diagnostic equipment in pathological labs and services will increase demand for in-vitro diagnostics, benefiting the growth of the industry as a whole.
Point-of-care (POC) Testing to Take Precedence over Centralised Laboratory Testing
Testing used to be done nearly entirely in centralised laboratories. For the diagnostic processes to operate as effectively and consistently as possible, centralised laboratory testing is crucial. In recent years, there has been a steady shift away from centralised laboratories to point-of-care testing services or decentralised clinical laboratory testing. This shift is primarily attributable to point-of-care testing's (POC) lower costs, convenience for patients and clinicians, shorter wait times for results, higher quality, and widespread use. POC tests can enhance patient care in situations involving infectious illnesses. Physicians can better manage clinical operations and keep an eye on patients receiving treatment for infectious diseases thanks to the decentralised.
In-Vitro Diagnostics Market Segment:
By Technique, in 2021, the molecular diagnostics market segment held the highest market share of 37.95% thanks to the launch of new products and continuous development in technology. Whereas complex equipment is often required for PCR, research efforts in this field have led to advancements such postage stamp-sized plasmofluidic chips. According to the ACS Nano Journal in May 2021, plasmofluidic chips are efficient and incredibly quick; they can complete a PCR test in just 8 minutes, and as a result, are anticipated to speed up diagnosis in both current and upcoming pandemics. Multiple launches were seen in the immunoassay market in 2021. This can be attributed to the COVID-19 pandemic's ongoing effects on the market and customers' increased demand. For instance, it can be challenging to distinguish between the flu and COVID-19 due to their comparable symptoms. The introduction of cutting-edge products that could identify both the flu and SARS-CoV-2 satisfied this unmet demand. To meet the demand, businesses like Roche have released the SARS-CoV-2 & Flu A/B Rapid Antigen Test on the market. For the purpose of identifying infectious microorganisms, tests and assays are included in the microbiology sector.
Regional Analysis:
- North America
- Europe
- Asia-Pacific
- South America
- Middle East and Africa
Speak with our research analyst:https://www.maximizemarketresearch.com/request-sample/362
About Us:
The Maximize Market Research team does B2B and B2C research on 12,500 high-growth technologies that present potential for organisations in the healthcare, pharmaceuticals, electronics, communications, internet of things, food and beverage, aerospace, defence, and other manufacturing sectors.
Contact us:
MAXIMIZE MARKET RESEARCH PVT. LTD.
3rd Floor, Navale IT Park Phase 2,
Pune Banglore Highway,
Narhe, Pune, Maharashtra 411041, India.
Get More Similar Link :
https://9020.social/blogs/15495/Audio-Equipment-Market-SWOT-Analysis-by-Size-Status-and-Forecast











